For over a decade persulfate has been used to oxidise contamination in the field of environmental remediation. Here, Regenesis discusses the development of its all-in-one oxidant product that employs advanced catalyst-based activation chemistry.
IN SITU chemical oxidation (ISCO) of groundwater and soil contaminants is a remediation approach widely practiced throughout the world.
The technique generally involves introducing a chemical oxidant (such as hydrogen peroxide, permanganate or persulfate) to the environmental media in such a way that there is direct contact between the oxidant and the target contaminant.
In the case of subsurface soil or groundwater treatment, this usually involves the injection of the oxidants into the subsurface in the form of an aqueous solution or slurry, or in the case of treating soils on the ground surface, mixing of oxidant powder or slurries into the soil.
Persulfate is considered a powerful direct oxidant as it has a theoretical standard oxidation potential of 2.0V (for the reaction S2O82- + 2e- →2SO42-). This is similar to that of ozone (2.1V) and greater than that of hydrogen peroxide (1.8V), sodium percarbonate (1.8V), and permanganate (1.7V). This strong oxidising potential of persulfate makes oxidation of many organic contaminants thermodynamically favourable.
Most persulfate project applications have involved the use of persulfate in conjunction with traditional activation chemistries which, while having been used successfully to degrade contamination in the field, have their own drawbacks in each case. Over the past decade, little has been accomplished toward improving the efficacy, cost effectiveness or occupational safety related to the use or persulfate oxidation chemistry for environmental remediation. However, one significant recent advancement has emerged in the form of a new all-in-one oxidant product that employs advanced catalyst-based activation chemistry.
Activation of persulfate
Although sodium persulfate has the ability to transfer electrons directly in the process of direct oxidation, where two electrons are transferred simultaneously as above, this reaction has long been shown to proceed at kinetically slow rates. In addition to the direct oxidation pathway, persulfate has the propensity to generate radicals. Radicals are generated when the persulfate anion reacts with another compound (or activator) to form atoms with unpaired electrons (radicals). Radicals are very reactive, rapidly oxidising other compounds (increased kinetic rates compared to direct oxidation). Thus it is generally accepted that the contaminant destruction kinetics achieved by activating the formation of radicals is far more suitable for ISCO than the kinetics of contaminant destruction through direct oxidation.
Development of catalysed persulfate
To overcome the cost, safety and/or efficacy issues associated with traditional activation techniques, a catalysed persulfate remediation technology has recently been developed by Regenesis R&D, and is sold by Regenesis under the trade name PersulfOx™. This all-in-one reagent provides an initial base-activation of persulfate, transitioning to catalysed contaminant destruction as the reaction proceeds. Since the catalyst is a true catalyst, it is not itself consumed by the reaction (in contrast to an activator), and is effectively ‘reused’ multiple times, enabling significant contaminant destruction from a relatively small quantity of catalyst material.
In this manner, a high proportion of oxidant material is maintained in the product (approx 90%), with powerful contaminant destruction catalysis and with no requirement to purchase, add or handle additional activation reagents. The product is packaged as an all-in-one fully water-soluble powder that is simply mixed with water and applied to the environmental medium to be treated.
The catalyst
The chemical basis of the PersulfOx catalyst is amorphous silica. Initially a water-soluble high pH material, as the pH drops during reaction, amorphous silica precipitates forming a very high surface area colloidal matrix. Amorphous silicas are strong adsorbants due to the surface silanol (-OH) groups, which weakly bind metals and metal oxides forming an heterogeneous catalyst and may themselves be radicalised.
The catalyst brings together both the contaminant and the oxidant and metal/metal oxide species in close proximity, thereby mediating reactions between the target contaminants, the oxidant, and free and/or bound radicals.
Field studies
PersulfOx laboratory and field studies have shown the destruction of contaminants to have similar effectiveness as traditional activation methods, without having to apply activator solutions. This results in a considerable potential saving in costs to the project, through eliminating supplementary reagent requirements and the associated fieldwork of moving, mixing and injecting the greater reagent volume.
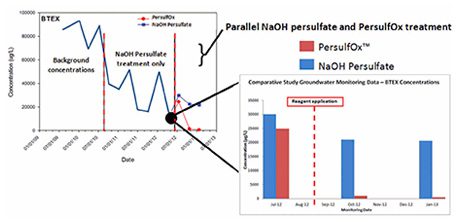
In an independent field study involving a side-by-side in situ treatment of BTEX and petroleum hydrocarbons in the C10 to C16 range, PersulfOx was found by McGregor (2013) to provide greater contaminant destruction than base-activated persulfate.
The study comprised side-by-side treatment of two sections of a longitudinally split plume which had previously received multiple applications of base-activated persulfate over a period of two years. During this period, persulfate treatment had not reached the remedial targets and was suffering from large contaminant concentration rebounds.
Where the two approaches were compared (see Figure 1), PersulfOx showed significant destruction and no sign of rebound occurring whereas base-activated persulfate showed only moderate declines from our equivalent oxidiser.
In situ chemical oxidation is an increasingly practiced remediation strategy with the potential to achieve rapid reduction of organic contaminants in soil and groundwater.
Persulfate, an oxidant used in environmental remediation, has historically been activated using the co-application of additional chemicals that are more costly to apply and can present significant safety hazards on-site.
Catalysed persulfate promises to lower the cost and improve the safety of persulfate-based remediation projects by employing an integrated catalyst.
Evaluation of PersulfOx suggests the performance to be close to or exceeding the top end of the range achievable through traditional activation approaches.







