A group claims to have developed a way to improve the quality of desalinated water while reducing the cost of the process. The findings were recently published in Proceedings of the National Academy of Sciences of the USA.
Desalination removes mineral particles (salts) from saltwater, making it fit for human consumption and for irrigation. Around 80% of drinking water in Israel, for example, is desalinated water, coming from the Mediterranean Sea.
This latest twist on the process has been developed by a group from the Technion-Israel Institute of Technology, Wageningen University, and Wetsus (the European centre of excellence for sustainable water) in the Netherlands.
The chemical properties of some mineral particles in saltwater make them more challenging to remove than others. Boron, which is naturally found in high quantities in the Mediterranean, is among the hardest to remove, as any change in acidity causes it to change its properties. It is toxic in high concentrations, and it harms plant growth, which is a problem with irrigation. The normal process of boron removal involves dosing the water with a base in order to facilitate removing the boron, followed by removal of the base.
Capacitive deionization
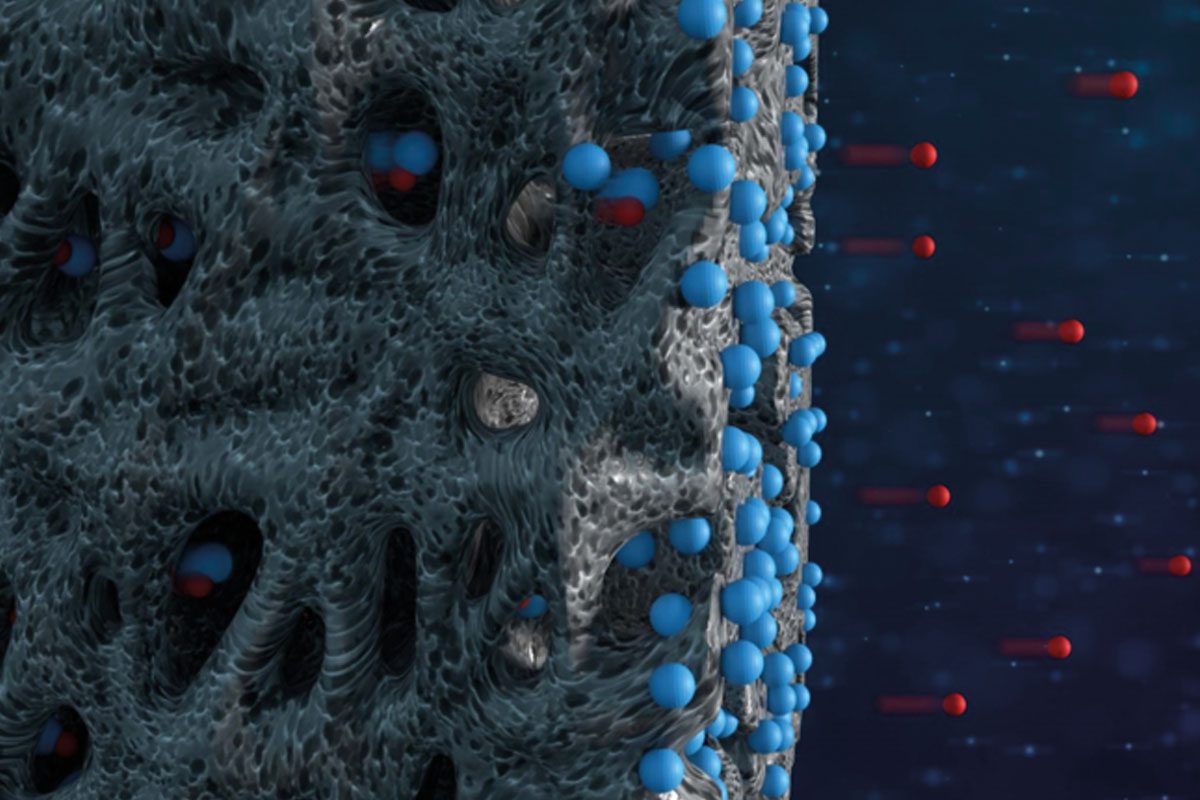
The most commonly used method of desalination is by means of a membrane – a sort of sieve that allows water to pass through it, while blocking other particles, based on their size or charge. This membrane, however, is expensive, and needs to be replaced periodically.
The group developed a new modeling technique to predict the behavior of boron during desalination by means of capacitive deionization. This is an emerging technique for water treatment and desalination using relatively cheap porous electrodes, as opposed to the expensive membrane. When an electric current is applied, charged particles (like boron under high pH conditions) are adsorbed by the electrodes and hence removed from the water.
PhD student Amit Shocron formulated the theoretical framework that allowed this breakthrough, while another PhD student Eric Guyes constructed the experimental setup. Working together, they were able to develop the novel system.
They found that for optimal boron removal, the positive electrode should be placed upstream of the negative electrode – counter to accepted wisdom in their field.
They also calculated the optimal applied voltage for the system, finding that higher voltage does not necessarily improve the system’s effectiveness.
This same method could be used to solve other water treatment challenges as well, says the group. For example, the removal of medicine residues and herbicides, which are difficult to remove using conventional methods.







