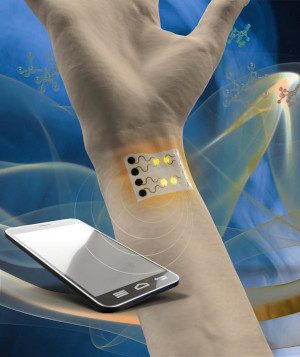
The notion of a sensitive, wearable gas sensor – equipped to perform environmental and human health monitoring – has been a technological grail for some time. Researchers at Penn State and Northeastern University say they are developing a platform that exhibits important advances in this effort.
The sensor device is an improvement on existing wearable sensors, says the group, because it uses a self-heating mechanism that enhances sensitivity. It allows for quick recovery and reuse of the device. Other devices of this type require an external heater. In addition, wearable sensors more often depend upon an expensive and time-consuming manufacturing process that uses lithography requiring cleanroom conditions.
“People like to use nanomaterials for sensing because their large surface-to-volume ratio makes them highly sensitive,” said Huanyu Cheng, assistant professor of materials science and engineering at Penn State University. The problem, he suggested, is “the nanomaterial is not something we can easily hook up to.” How do you attach wires to the material, for example, to receive the signal? This requires the use of interdigitated electrodes, which are individually addressable, comb-like electrode structures – a widespread fixture in biological and chemical sensors given their inexpensive and relatively easy fabrication process, and high sensitivity. The presence of a biological or chemical analyte can be detected via the change it causes in the impedance or capacitance at the electrodes, which is measurable.
Cheng and his team use a laser to pattern a highly porous single line of nanomaterial similar to graphene for sensors that can detect gases, biomolecules, and chemicals. In the non-sensing portion of the device platform, the team created a series of serpentine lines coated with silver. When an electrical current is applied to the silver, the gas sensing region heats up given its significantly greater electrical resistance, eliminating the need for a separate heater. The serpentine lines allow the device to stretch, like springs, to accommodate the flexing of the body as the wearer moves.
The nanomaterials used in this work are reduced graphene oxide and molybdenum disulphide, or a combination of the two. Another option is a metal oxide composite comprising a core of zinc oxide and a shell of copper oxide – the two classes of widely used gas sensor material.
“Using a CO2 laser, often found in machine shops, we can easily make multiple sensors on our platform,” Cheng said. They plan to incorporate in the region of tens to a hundred different sensors, each selectively tuned to a different molecule. The end result would be “an electronic nose able to decode multiple components in a complex mixture.”
The US Defense Threat Reduction Agency is interested in this wearable sensor to detect chemical and biological agents that could damage the nerves or lungs, according to the researchers. A medical device company is also working with the team to scale up production for patient health monitoring, including gaseous biomarker detection from the human body and environmental detection of pollutants that can affect the lungs.
Ning Yi, a doctoral student in Chen’s lab and co-lead author of the paper posted online in the Journal of Materials Chemistry A, said, “In this paper, we showed that we could detect nitrogen dioxide, which is produced by vehicle emissions. We can also detect sulphur dioxide, which, together with nitrogen dioxide, causes acid rain. All these gases can be an issue in industrial safety.”
The researchers said their next step is to create high-density arrays and try some ideas to improve the signal and make the sensors more selective. This may involve using machine learning to identify the distinct signals of individual molecules on the platform.







